Is vendor qualification creating unnecessary inefficiency in your organization? You’re not alone. Sponsors have historically relied on independent auditors to assess the same Vendors repeatedly, draining time and resources.
Diligent Pharma offers a streamlined, modern solution that simplifies the Vendor qualification process while maintaining the highest standards of quality and compliance. With our centralized platform, Sponsors can easily access comprehensive audit reports, submit qualification requests, and manage clinical trial Vendor relationships.
Innovation in highly regulated industries often comes with questions, and we’re here to provide the answers. In this blog, we’ll address some of the most frequently asked questions (FAQs) about how we manage vendor qualification for clinical trials, what’s involved in a typical audit, and how you can apply the Diligent model to fit your unique needs. Let’s dive in.
What does it mean to “qualify” a clinical trial Vendor, and why is it necessary?
“Qualifying” a Vendor means conducting a thorough assessment and verification process to ensure they have the required expertise, infrastructure, and compliance processes to deliver the necessary services or technology for a clinical trial. According to ICH E6(R3) guidelines, Sponsors are responsible for selecting and evaluating clinical trial Vendors to ensure they can competently and compliantly perform the outsourced tasks or activities. This process guarantees that Vendors meet regulatory standards and operate in accordance with Good Clinical Practice (GCP) and Good Clinical Laboratory Practice (GCLP) requirements.
Our independent, expert-led audits empower Sponsors to verify Vendor capabilities, infrastructure, and processes, ensuring compliance with global regulatory requirements and GCP/GCLP guidelines. Our centralized platform represents a landmark innovation in the Vendor qualification process, which is fully aligned with the ICH Vendor Risk Management Framework.
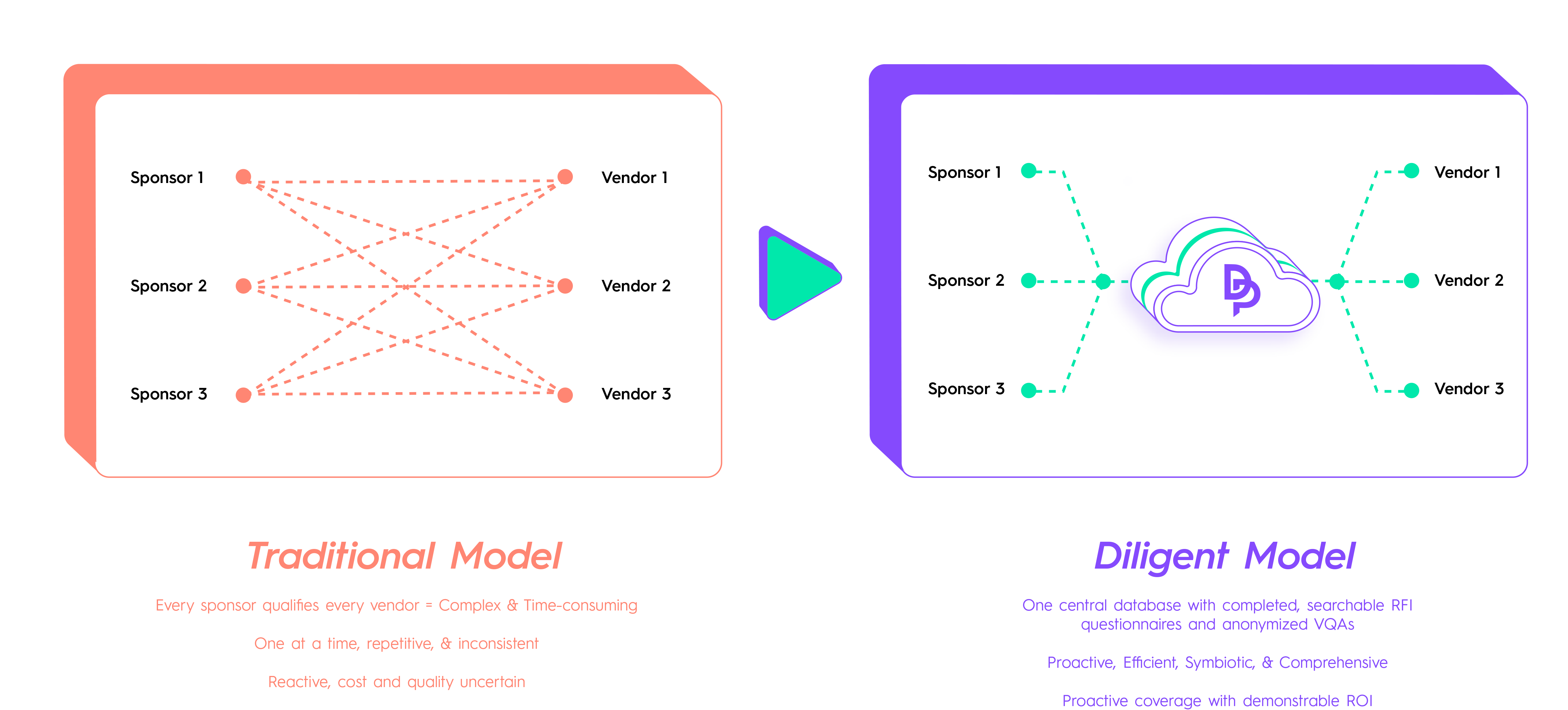
How does Diligent work?
Our team uses a rigorous, standardized process to qualify clinical trial Vendors. The result is a comprehensive Qualification Package that includes the audit report and completed Qualification Questionnaires (RFIs).
Vendors can securely manage this package on our platform and share it with interested trial Sponsors within the Diligent Platform. Sponsors can easily search for Vendors, submit confidential requests for their qualification package, and, upon Vendor approval, access the detailed data needed to complete their qualification process more efficiently and confidently.
What is included in a standard audit scope?
Every audit assesses essential core capabilities. Here is what must be included when performing vendor qualification for clinical trials.
Organization
• Ensuring that the Vendor is financially capable of supporting clinical trial needs
• Checking that the Vendor adheres to required insurance standards and policies
• Evaluating compliance with ethical standards, including anti-bribery and anti-corruption policies
Privacy & Personal Data Protection
• Ensuring that systems meet FDA regulations for electronic records
• Checking for compliance with GDPR for handling personal data
• Ensuring adherence to HIPAA laws in the U.S. for privacy and data security
Quality Management Systems
• Assessing if the Vendor has a system in place to manage quality and compliance with respect to regulatory requirements
• Evaluating how the Vendor manages and oversees quality in third-party relationships
• Ensuring proper management and control of documentation
Operations & Project Management
• Checking processes for secure and efficient data handling and transfer
• Reviewing human resources practices, including staffing and employee training
• Assessing the Vendor’s management of physical facilities
Why trust the quality of Diligent’s work?
Diligent’s audit team is composed of seasoned professionals, each with over ten years of GCP or GCLP experience and the technical expertise needed for thorough Vendor qualification assessments. Our clients include several of the top ten pharmaceutical companies known for their stringent quality requirements. These companies trust our FDA-accepted cooperative audit model, which meets and often exceeds their internal standards for Vendor qualification. Our thorough processes and detailed audit reports ensure the highest quality and reliability.
How is confidentiality and data protected?
Clinical trial Vendors maintain full control over their audit reports and RFIs, and no data is shared without their explicit approval. When a Sponsor submits a request, the Vendor must approve it before any information is released. Only the Vendor’s name, service offerings, and available data are visible on their profile. Sponsors cannot see who else has requested or accessed the Vendor’s information, ensuring complete confidentiality in the process.
Has the FDA reviewed Diligent’s model?
Yes, the FDA has reviewed Diligent’s centralized model and has deemed it compliant for use by pharmaceutical and biotech companies. The FDA accepts our cooperative audit approach as a valid method for qualifying clinical trial Vendors, making it a credible and compliant solution for Sponsors. Several of Diligent’s clients have undergone FDA inspections with zero findings related to their use of Diligent’s platform or audit reports. Furthermore, our model has proven successful during the New Drug Application (NDA) and Biologic License Application (BLA) submission process, holding up to FDA scrutiny throughout regulatory reviews.
How is leveraging centralized qualification data a credible and acceptable way to qualify clinical trial Vendors?
According to ICH Q9, ICH Q10, and ICH E6(R3), the qualification method is left to the discretion of the trial Sponsor. The industry has long relied on independent auditors to assess and qualify the same Vendors time and time again. Diligent’s platform allows Sponsors to leverage a single, rigorous qualification for multiple Sponsors, eliminating the redundancy of conducting nearly identical audits in most cases. This streamlined approach maintains credibility, as it’s built on industry standards and the independent audit process that Sponsors have trusted for years.
How can I apply the Diligent model to my organization?
Diligent’s model is designed to be flexible, allowing you to use it where it best supports your needs. Whether you’re just beginning to identify potential clinical trial Vendors or already have a list and need help with audits, Diligent can seamlessly integrate into your process. You can start with early-stage discovery, where we help you gather and refine RFI data, or jump directly into risk characterization to evaluate potential Vendor threats.
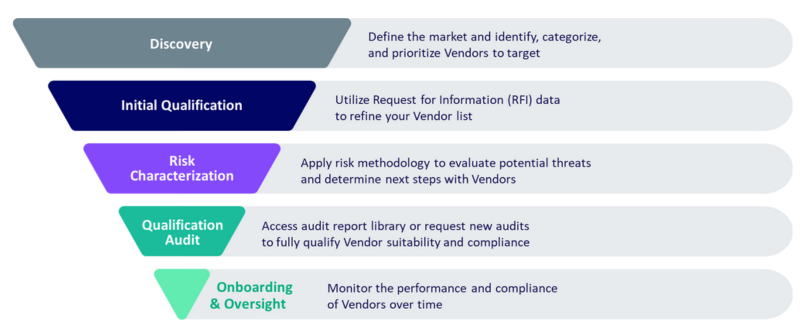
If your focus is on audit qualification, Diligent offers access to a robust library of audit reports. We also allow trial Sponsors to request new audits for specific Vendors or join upcoming scheduled Vendor qualification audits. Once onboarded, our platform also provides ongoing oversight, ensuring your Vendors maintain compliance. You decide how and when to leverage Diligent’s expertise, making the process as comprehensive and targeted as needed.
Proven. Vetted. Diligent. Explore the Diligent Qualified Library— new Vendor audit reports and RFIs are added to our library every week.
Questions? Let’s chat.
Schedule a one-on-one with our team to learn more about how Diligent can support your unique qualification and risk management needs.
